FDA FHIR Pilot Automates Adverse Events Reporting and Validation with Cedars-Sinai and the VA Through eHealth Exchange
Healthcare IT Today
SEPTEMBER 20, 2024
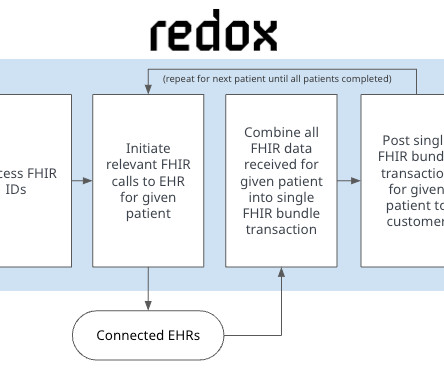
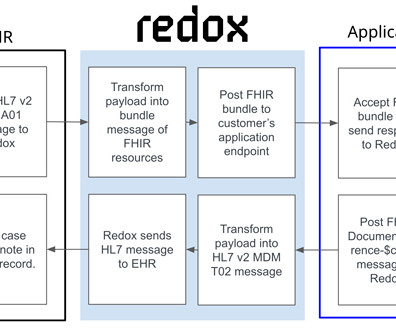
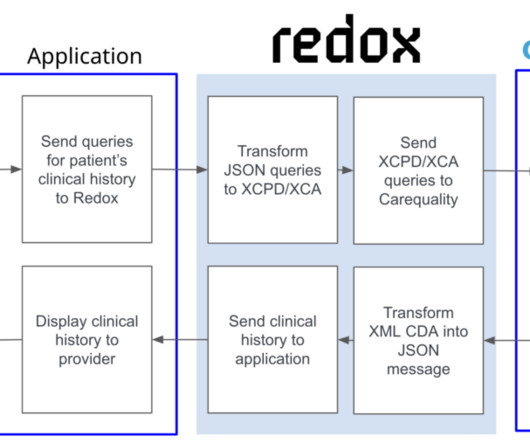
The goal was to enable clinicians to report adverse events directly from their electronic health records (EHRs) in a highly automated manner. The decision to leverage HL7’s FHIR Release 4 is important for the long-term viability of this project as it expands to include reporting for other drugs and use cases.








































Let's personalize your content